NuCLEANase receives positive GRAS evaluation from US FDA
Author / c-LEcta
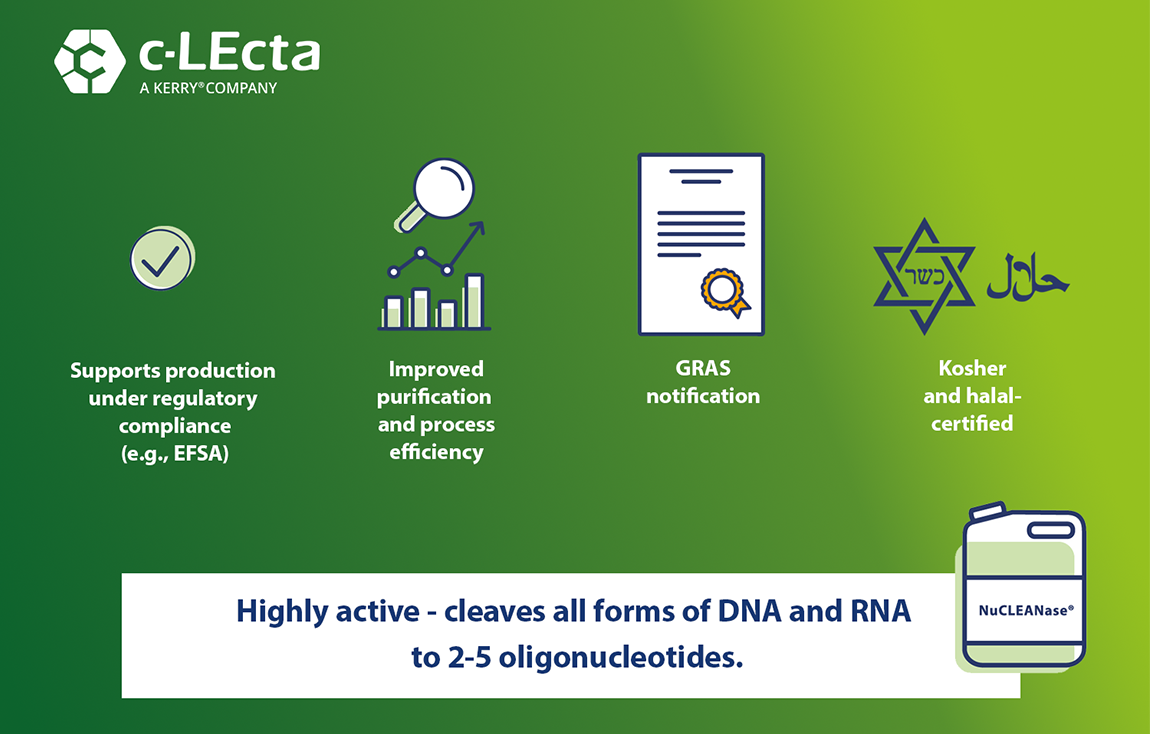
After submitting a GRAS notification for NuCLEANase (GRN No.1146) to the US FDA, c-LEcta has received a positive evaluation with no questions from the authority, which is publicly available within the GRAS Inventory List. The GRAS Notification designates that the usage of NuCLEANase in food applications is considered safe under the conditions of its intended use.
As NuCLEANase is intended for the hydrolysis of nucleic acids during manufacturing of microbial-derived ingredients, the processing aid may be applied for various purposes such as the aim of meeting regulatory requirements for the absence of DNA, help to reduce the viscosity of the fermentation broth and to increase the efficiency of the recovery process.